目次
[Graphic explanation] Yuzu aroma component – structural formula –
Hello. I’m Fig working in a flavor and fragrance industry for about 10 years.
Yuzu has nice aroma, Japanese use it at cooking. Do you know what Yuzu aroma components are?
Yuzu has more than 75 aroma components. Especially the followings are key components of Yuzu aroma.
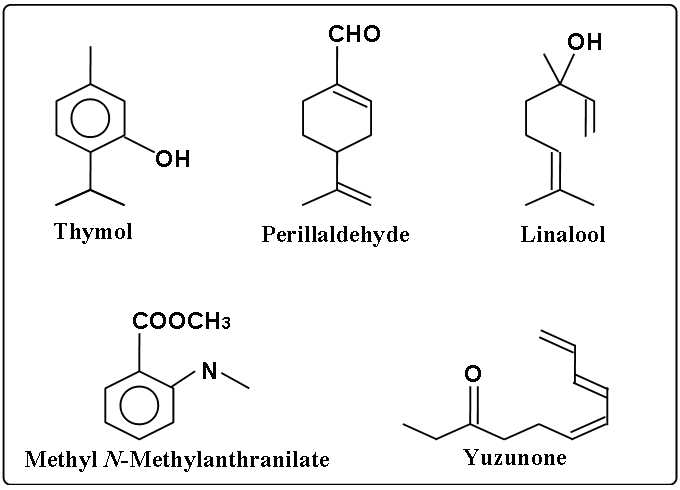
- Thymol
- Perillaldehyde
- Linalool
- Methyl N-Methylanthranilate
- Yuzunone
In case of citrus oil (Yuzu, orange, lemon and so on), there is 80% limonene. 20% components decide the characteristic.
 [Graphic explanation] Citrus aroma comparison – orange, lemon, lime –
[Graphic explanation] Citrus aroma comparison – orange, lemon, lime –
When you know the molecular shape in detail, you can easily image aroma components. Let’s check these structural formula and pictures of molecular model.
Thymol
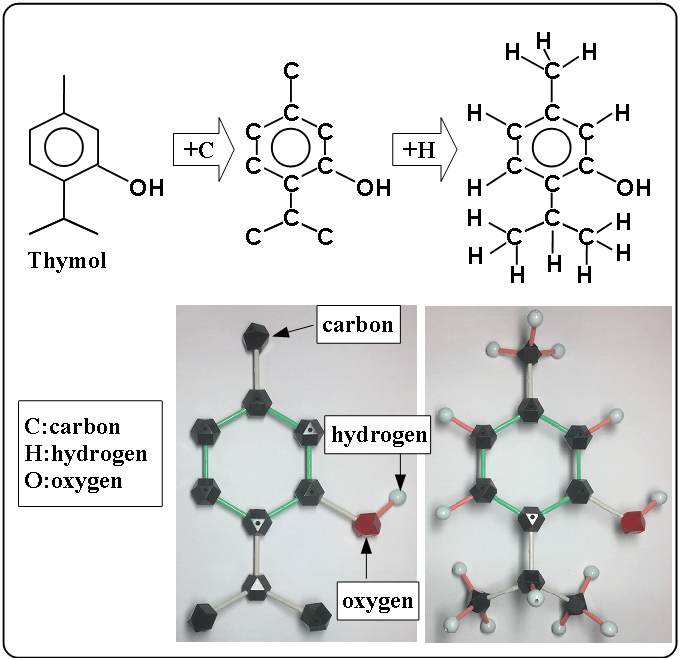
The left figure is a simple structural formula. The middle figure is added carbon, and the bellow is a pictures of molecular model. In molecular model, the black block shows carbon and the red shows oxygen and the light blue shows hydrogen. The right figure is added hydrogen. The right figure is more complicated than the left. It is easy for chemists, especially organic chemists, to image a molecular shape with a structural formula.
A molecule is three-dimensional, it can be rotated as the following.
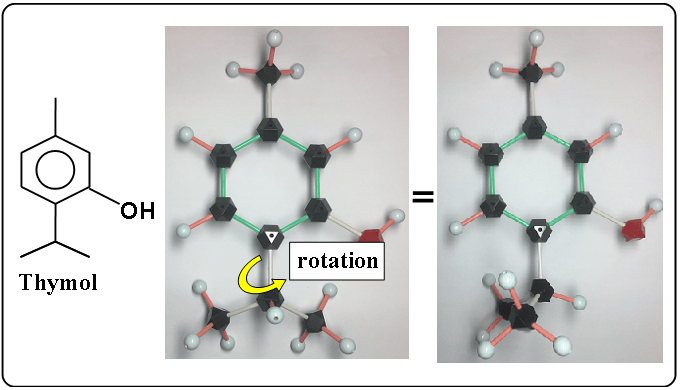
The both molecular models are thymol.
Perillaldehyde
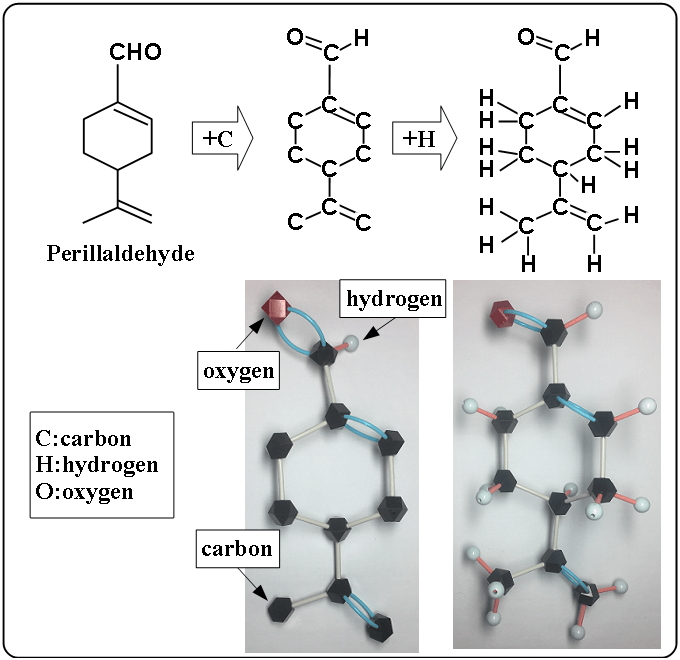
The left figure is a simple structural formula. The middle figure is added carbon and the right figure is added hydrogen. The bellow pictures are molecular models. In molecular model, the block color meas as the following.
- black : carbon
- red : oxygen
- light blue : hydrogen
Compared to thymol molecular model, the hexagon part of perillaldehyde is not regular hexagon because of different carbon-carbon bonds. But in structural formula, the part is shown as a regular hexagon.
Linalool
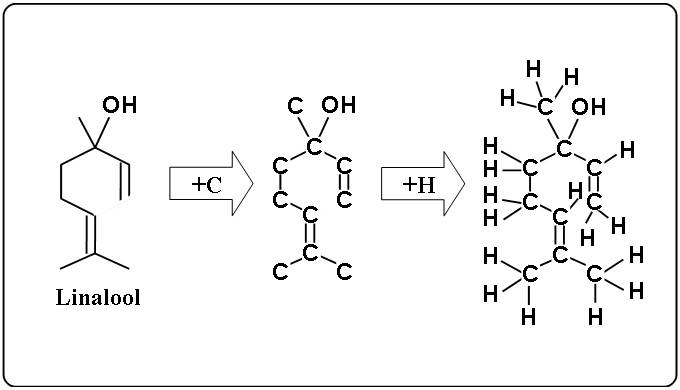
- left figure : a simple structural formula
- middle figure : added carbon
- right figure : added hydrogen.
Two kinds of linalool
There are two kinds of linalool as the following.
- d – linalool (lavender-like)
- l – linalool (petitgrain-like)
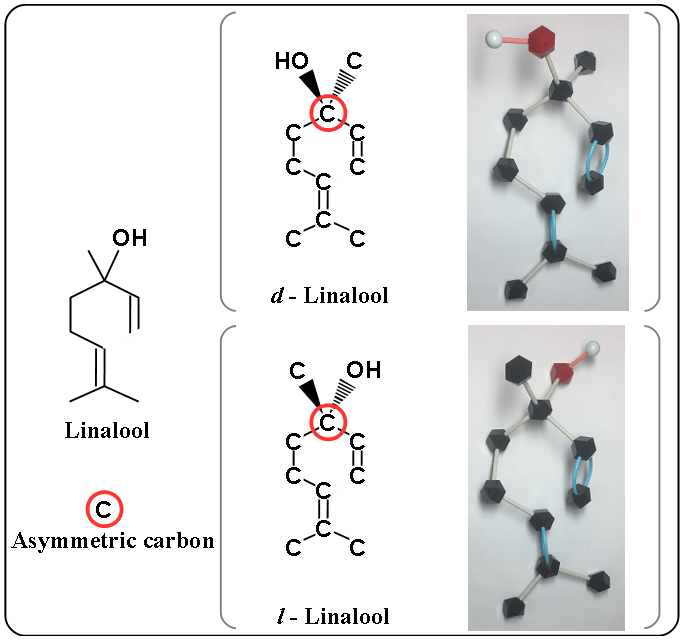
The difference is caused by the carbon of red circle (asymmetric carbon). A bold-wedged line indicates that the bond is protruding out from the plane of the drawing surface. A dashed line indicates that the bond is extending behind the plane of the drawing surface. In molecular models, the oxygen (red block) is upward in d – linalool and downward in l – linalool.
The structural formula added hydrogen is shown as the following.
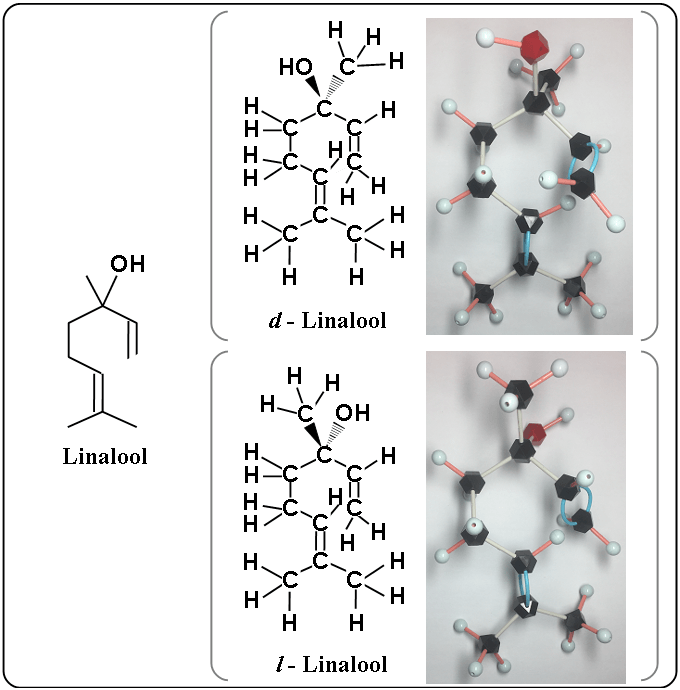
And let’s compare d – linalool with l – linalool.
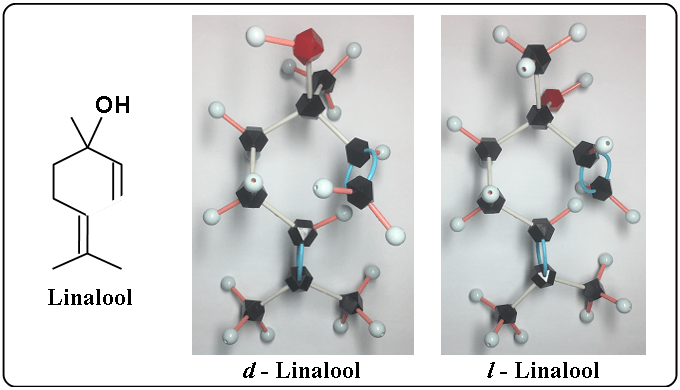
By the little difference, the different scents are caused in linalool.
Methyl N-Methylanthranilate
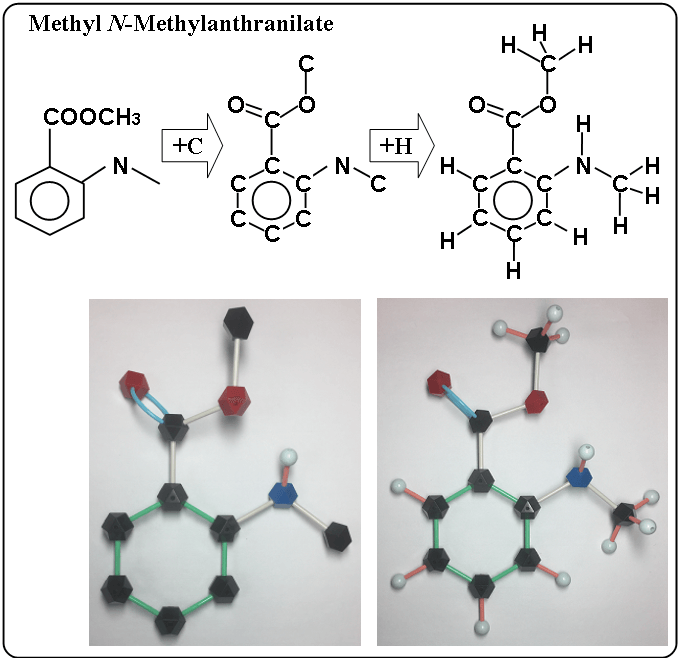
- left figure : a simple structural formula
- middle figure : added carbon
- right figure : added hydrogen.
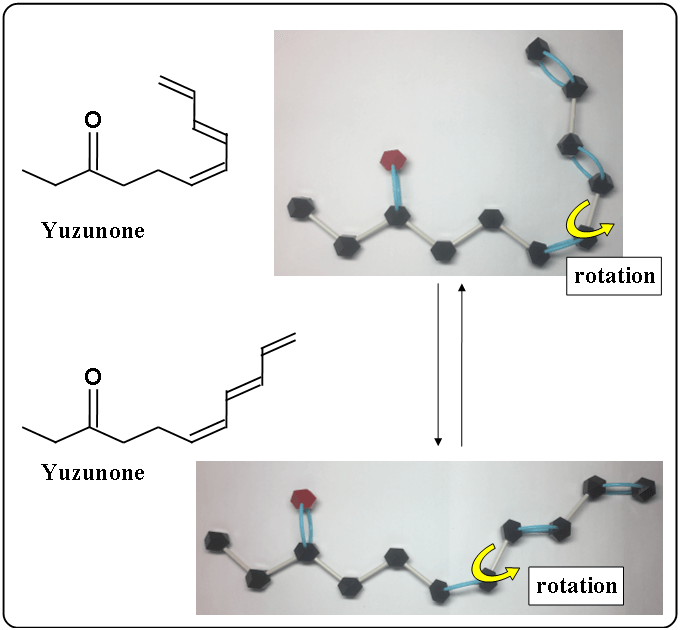
Yuzunone
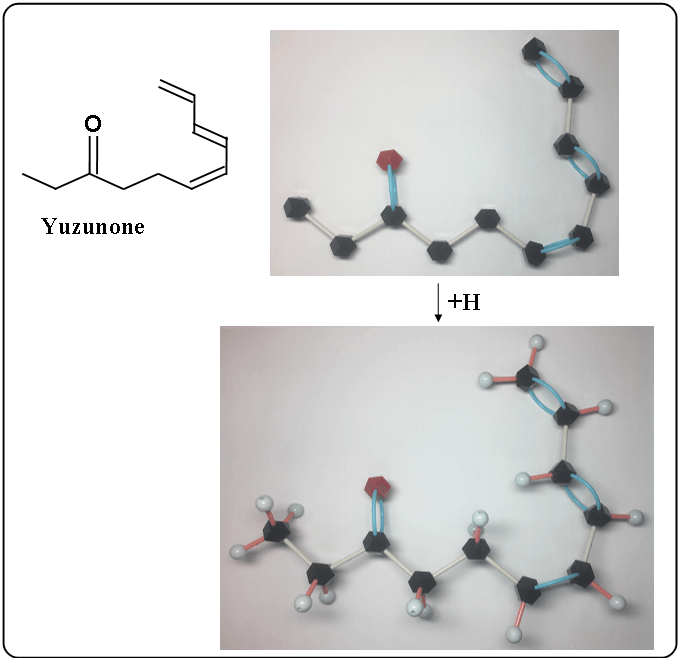
Yuzunone is a chain molecule. It can be rotated as the following.
Summary

- Thymol
- Perillaldehyde
- Linalool
- Methyl N-Methylanthranilate
- Yuzunone
These are key components of Yuzu aroma.
In the following page, you can know how to get the essential oil, production of Yuzu.
 [Graphic explanation] Yuzu essential oil – crop season, production, extraction –
[Graphic explanation] Yuzu essential oil – crop season, production, extraction –
References
(1) T. HASEGAWA CO., LTD. Koryo no Kagaku (Science of Flavor and Fragrance) Kodansha (2013).
(2) Nippon Koryo Kyokai Kaori no Hyakka (Encyclopedia on Scent) Asakura Shoten (1989).
(3) David G. Williams. The Chemistry of Essential Oils Translated by Takeo Kawaguchi. Fragrance Journal (2013).
(4) The Chemical Society of Japan ed. Koryo no Kagaku (Chemistry of Flavor and Fragrance) Dainippon tosho (1983).
 図解アロマ
図解アロマ 

